COVID-19 / Coronavirus
$60.00
Description
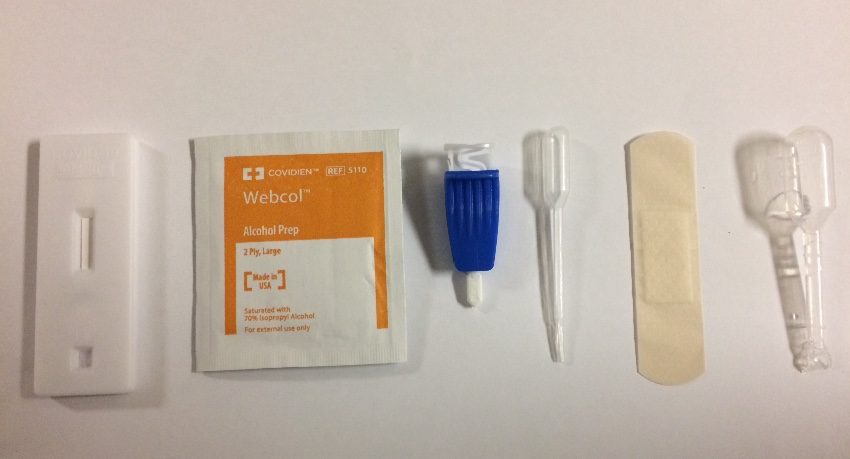
COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) with one drop of serum/plasma specimen, this test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) must be confirmed with alternative testing methods(s) and clinical findings.
CLICK ON THE LINK BELOW TO VIEW A “HOW-TO VIDEO” TO SEE HOW THE COVID-19 RAPID TEST CASSETTE WORKS:

MISSION STATEMENT FOR COVID-19 PANDEMIC
In the hope of helping in the fight against this pandemic our company has increased the number of tests performed in order to decrease the alarming death rate in the world by making COVID-19 testing available and affordable to everybody on the planet. This preventive approach of aggressive cost efficient testing with very sensitive and accurate tests in tandem with “social distancing” and “contact tracing” have proved to decrease the “pre-symptomatic infection” rate by 80% in Asia because of faster turnaround time of 10 to 15 minutes instead of waiting from 1 to 2 weeks before you can get a result back from the majors labs. With the exponential growth of new cases it doesn’t take a rocket scientist to figure out that if we can catch only “ONE” asymptomatic COVID-19 patient with these rapid testing kits we have averted 1000 new case and thereby prevented 10 death if the death rate is only one percent.
WHAT IS CORONAVIRUS DISEASE (COVID-19)?
COVID -19 is the disease caused by the new coronavirus that emerged in Wuhan China in December 2019. COVID-19 spreads between people who are in close contact (within about 6 feet) through respiratory droplets produced when an infected person coughs, sneezes, or talks. Recent studies indicate that people who are infected but do not have symptoms likely also play a role in the spread of COVID-19.
*Note: Older adults and people of any age with certain serious underlying medical conditions like lung disease, heart disease, or diabetes are at higher risk for developing more serious complications from COVID-19 illness and should seek care as soon as symptoms start.
Watch for symptoms
Reported illnesses have ranged from mild symptoms to severe illness and death for confirmed coronavirus disease 2019 (COVID-19) cases.
These symptoms may appear 2-14 days after exposure (based on the incubation period of MERS-COV viruses).
COVID-19 symptoms include:


- Cough
- Fever
- Shortness of breath
- Muscle aches
- Sore throat
- Unexplained loss of taste or smell
- Diarrhea
- Headache
- Shortness of breath
- COVID-19 can lead to severe respiratory problems, kidney failure or death.
For the latest information, visit the CDC website at www.cdc.gov/coronavius/2019-ncov. Or call 800-CDC-INFO (800-232-4636).
How is COVID-19 Diagnosed?
Diagnosis may be difficult with only a physical exam because mild cases of COVID-19 may appear similar to the flu or a bad cold. A laboratory test can confirm the diagnosis. Learn more about COVID-19 testing
Your healthcare provider will ask about your symptoms. He or she will also ask about your recent travel and contact with sick people. If your healthcare provider thinks you may have COVID-19, he or she will work closely with your local health department or testing. Follow all instruction from your healthcare provider. COVID-19 is diagnosed by:
- COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) with one drop of serum/plasma specimen, this test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) must be confirmed with alternative testing methods(s) and clinical findings.
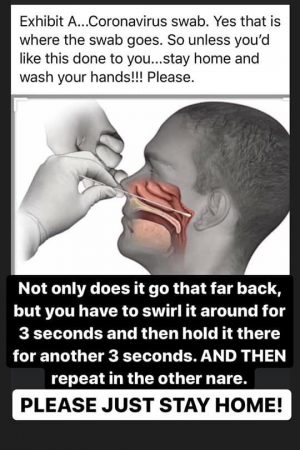
- Nose and throat swab. A cotton tipped swab is wiped inside your nose or throat. This is done to check for viruses in your nasal mucus.
- Sputum culture. A small sample of mucus coughed from your lungs (sputum) is collected.
-
What to do after you are tested
- If you test negative for COVID-19, you probably were not infected at the time your specimen was collected. However, that does not mean you will not get sick. It is possible that you were very early in your infection when your specimen was collected and that you could test positive later. Or you could be exposed later and then develop illness. In other words, a negative test result does not rule out getting sick later.
- If you test positive for COVID-19, know what protective steps to take If You Are Sick or Caring for Someone.
When to Seek Medical Attention
If you develop emergency warning signs for COVID-19 get medical attention immediately. Emergency warning signs include*:
- Trouble breathing
- Persistent pain or pressure in the chest
- New confusion or inability to arouse
- Bluish lips or face
- *This list is not all inclusive. Please consult your medical provider for any other symptoms that are severe or concerning.
Call 911 if you have a medical emergency: Notify the operator that you have, or think you might have, COVID-19. If possible, put on a cloth face covering before medical help arrives.
Take everyday preventive steps
- Wash your hands frequently
- Avoid touching your eyes, nose, and mouth.
- Stay home when you are sick.
- Cover your cough or sneeze with a tissue, then throw the tissue in the trash.
- Clean and disinfectfrequently touched objects and surfaces
CLICK ON THE LINK BELOW AND ENTER OUR CLIA #: “10D2102167”
TO CHECK IF “PRIMARY DIGNOSTICS LABORATORY PLLC” IS CLASSIFIED AS ONE OF THE LABS THAT CAN PERFORM THE COVID-19
TEST:
https://www.cdc.gov/clia/LabSearch.html
Delivery time: Next day delivery for single online order and 3 to 7 day for bulk shipping.
Details:
FDA EUA pending, authorized to distribute
Results in 10 minutes at point-of-care
Detect IgM & IgG antibodies to COVID-19, in asymptomatic individuals, months post-infection
No special skill or equipment required
Works with whole blood, plasma, or serum samples
Demonstrated 95% Positive Percent Agreement, 94% Negative Percent Agreement in clinical studies.
Ranked 2nd out of 9 commercially-available immunoassay kits tested in an independent, published clinical study by Denmark government
Clinically validated by multiple CDCs and independent third parties
Over 2 million sold to government agencies and private organizations across the world with no adverse reporting
PLEASE UNDERSTAND THAT IF THE THIS SCREENING TEST IS POSITIVE IT WILL NEED TO BE CONFIRM WITH A MORE SPECIFIC TEST USING A SWAB FOR THE COLLECTION OF THE SPECIMEN AS FOLLOW IN THESE VIDEOS:
Video of self-collecting the swab for collection.
MOST PEOPLE ARE NOT INTERESTED IN GETTING TESTED BECAUSE OF IMAGE LIKE THIS PICTURE BELOW:
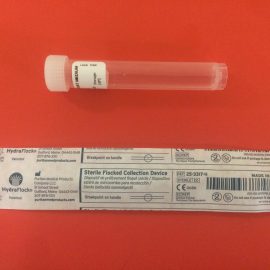
BECAUSE OF PROBLEMS THAT THE HEALTH CARE PROVIDERS WERE EXPERIENCING AT HOME THE FDA HAS APPROVE A IN-HOME COLLECTION KIT FOR THE CORONAVIRUS. PLEASE CLICK ON THE LINKS BELOW TO GET MORE INFO ABOUT THIS COLLECTION KIT AND HOW YOU CAN GET IT DELIVER TO YOUR HOUSE:
https://www.nytimes.com/2020/04/21/health/fda-in-home-test-coronavirus.html
https://www.pixel.labcorp.com/sites/default/files/covid-19-collection-instructions.pdf?v=1

Comparison of Swab and Antibody Testing
When you are infected by a virus, the virus hijacks your cell, copies itself hundreds of times, and then blows up your cell and spreads to other cells. It is your immune system’s job to stop the virus before it causes too much damage. Your body uses different techniques to slow down and kill the virus, like producing mucus, releasing chemicals and antibodies, and raising your body’s temperature. You’re alive today because your body has successfully killed billions of viruses during your lifetime. But every once in a while, a virus comes along that is difficult to fight. Coronavirus is one of them.
Figure 1. PCR tests use swabs to collect samples from the respiratory tract.
Swab tests use swabs to collect a sample from your nose or throat. They detect the presence of the virus’s genetic material in the sample. Swab tests are often called PCR tests by the scientific and medical communities, referring to the scientific method behind the test. PCR stands for “Polymerase Chain Reaction,” which is a method used by scientists to amplify the genetic material so it can be detected. It’s like a copy machine — it’s hard to see one sheet of paper from the side, but easy to see thousands of sheets. PCR tests work really Coronavirus well for about the first week after you start to feel sick. But as your body kills the virus, the test doesn’t work as well because there is no more virus around to detect. This is where your antibodies come in very handy.
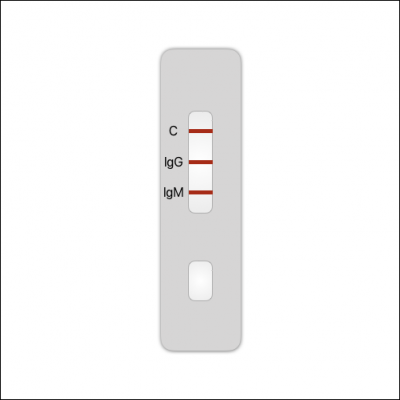
Figure 2. Antibody tests use a cassette like this. A blood drop is collected and added to the sample well. The result is read 10-20 minutes later.
Antibody tests use a blood sample to detect the presence of antibodies produced in response to the virus. For people with strong immune systems, antibodies start floating around in your blood in detectable levels as early as 2-5 days after symptoms begin but can take as long as 10-14 days for people with weaker immune systems. Generally, the older a person is, the longer it takes for their body to respond, which is why coronavirus can be especially harmful to the elderly.
Types of Antibodies. Most commercial antibody tests detect two antibodies. Immunoglobulin M (“IgM” for short) antibodies are produced first in response to a virus but then disappear after a few weeks. Immunoglobulin G (“IgG” for short) antibodies are produced after a week or two but stay around for a long time after the infection is gone. This is helps us figure out if a person was infected weeks or even months earlier. If your body has IgG antibodies and it encounters the same virus again, it is usually able to attack and kill it quickly, before you even get sick again.
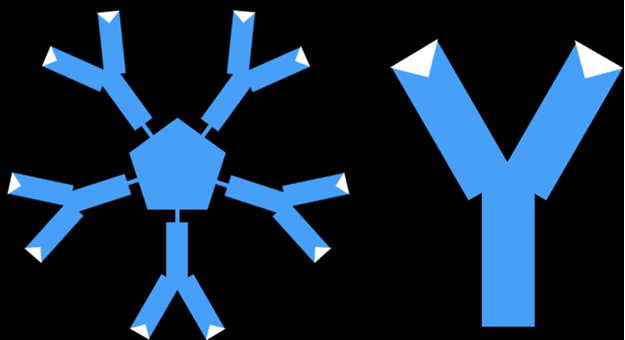
Figure 3. IgM and IgG antibodies.
PCR vs Antibody Tests. Contrary to some information surrounding PCR vs. Antibody tests, one test is not better or more sensitive than the other; they each provide valuable information and more reliable results at different points of time in the infection lifecycle. On average, after around Day 8 of the infection, antibody tests become more sensitive than PCR tests. By Day 14, PCR tests usually become very unreliable because the virus is mostly gone. Each test can give false positive or false negative results if samples are not collected properly, tests are performed incorrectly, or they are done at the wrong time.
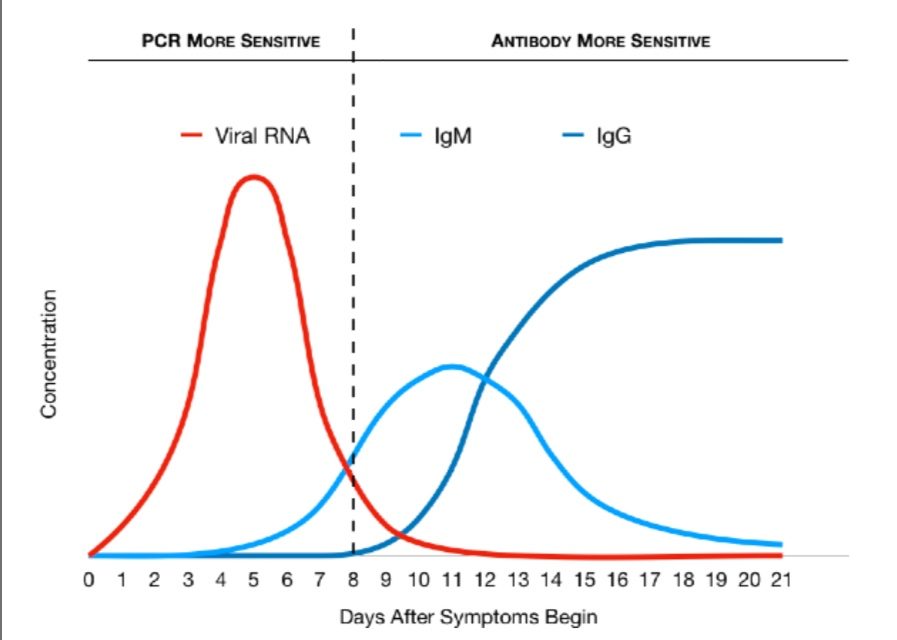
Graph 1. Relative levels of viral RNA and antibodies during infection lifecycle.
Timing is Extremely Important. Because PCR and Antibody tests give optimal results during timeframes of infection and symptom onset, it is important to understand when to use each test. Using the wrong test at the wrong time will result in inaccurate results even if the tests themselves have high accuracy and sensitivity.

Table 1. When PCR and Antibody are best used. These are general guidelines. Physicians should use their own discretion based upon information available on a case-by-case basis.
Individual Variability Must Be Considered. Individual immune systems respond differently depending on age and other health factors. The diagrams and recommendations below are based on general findings of clinical research. Immunocompromised individuals may not develop antibodies for a longer period of time. In such cases, PCR tests would yield more accurate results for severe, active infections. During the time period when viral RNA is decreasing and antibodies are increasing (typically days 6-10), it may be prudent to perform both tests since either could produce false negatives due to low concentrations of viral RNA or antibodies. In fact, a recent study found that combining RNA and antibody tests significantly improved the sensitivity of diagnosis for COVID-19 patients, even within the first week of symptom onset. The study also found that a higher concentration of antibodies was associated with a worse health condition.
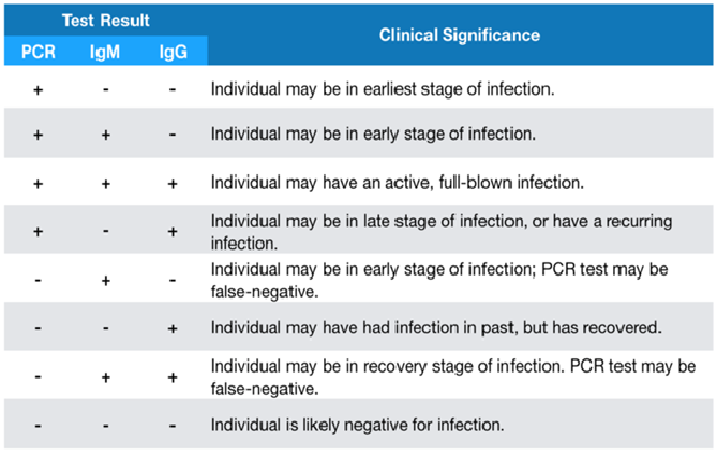
Table 2. Clinical significance of combined PCR and Antibody test results.
New research suggests that physicians should consider administering both swab and antibody tests to more accurately diagnose COVID-19, and to obtain information about the severity and timing of the infection.
Study reference: Juanjuan Zhao Jr. et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Medrxiv (pre-print). March 2019. doi: https://doi.org/10.1101/2020.03.02.20030189
About Our Coronavirus (COVID-19) Instant Test Kits for Medical Professional Use
Rapid results in 10 minutes
_Small sample sizes
_Sold in packs of 100
_Following the incubation period, IgM may appear in blood within 3-5 days. IgG will appear as soon as 1-2 weeks.
_Shelf life of 24 months from manufacture date
_Forensic / Medical Professional Use Only
_Tests should be conducted by a licensed phlebotomist, or a medical professional
_Verification of use case prior to shipping is mandatory
COVID-19 IgG/IgM Rapid Test Cassette
The test is conducted by using whole blood/serum/plasma and placing it in the center well of the cassette and then adding two drops of the buffer to the buffer well labeled “B.” Results should appear within 10 minutes and are invalid after 15 minutes. For more information, watch our video.
Clinical Evaluation Results:
The clinical evaluation was carried out during February 2020 across 10 hospitals and reported on March 12, 2020. Further clinical evaluations are ongoing and any further information needed can be granted at request.
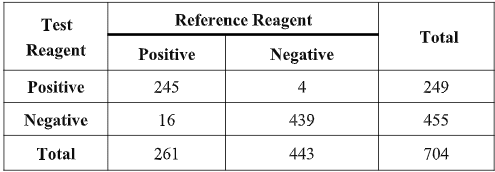
The results show that the testing reagent and reference reagent have equivalent effectiveness in detecting COVID-19 when tested in the same clinical specimens. Compared with the reference reagent, the positive agreement was 93.87% (95%CI:90.24%~96.46%), the negative agreement was 99.10% (95%CI:97.70%~99.75%) and total agreement was 97.19% (95%CI:95.65%~98.26%). The kappa value of the consistency analysis was 0.94 (95%CI:95.65%~98.26%). The results of the clinical evaluation show that the two reagents (methods) have a high degree of consistency and equivalent sensitivity and specificity in detecting COVID-19.
Product Facts:
Following the incubation period, IgM may appear in blood within 3-5 days. IgG will appear as soon as 1-2 weeks.
Sample: Test can work with whole blood, plasma and serum samples.
Storage: The kit can be stored at room temperature or refrigerated (2-30°C).
Shelf Life: 24 months from manufacture date
More information, such as the product insert, clinical trial results, and CE documentation is available at request
More detailed results of ongoing clinical evaluations and test stability report are available upon request.
COVID-19 IgG/IgM Rapid Test Cassette Instructions for Use

Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
Place the test device on a clean and level surface.
(For Whole Blood Specimen): Hold the 5 μL mini plastic dropper vertically and transfer 1 drop of whole blood (about 10 μL) to the specimen well(S) of the test device, then add 2 drops (about 80 μL) of sample buffer to the buffer well (B) immediately. Avoid air bubbles.
(For Serum or Plasma Specimens): With a 5 μL mini plastic dropper provided, draw serum/plasma specimen to exceed the specimen line as showed in the following image and then transfer drawn serum/plasma specimen into the sample well (S). Then add 2 drops (about 80 μL) of sample buffer to the buffer well (B) immediately. Avoid air bubbles.
Wait for the colored line(s) to appear. The result should be read in 10 minutes. Positive results may be visible as soon as 2 minutes. Do not interpret the result after 15 minutes.
Interpretation of Results:
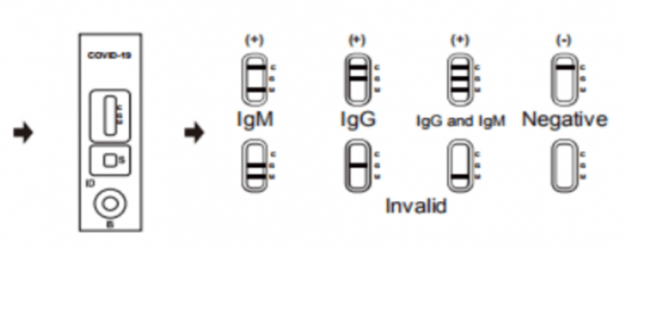
Negative: If only the C band is present, the absence of any burgundy color in the both T bands (IgG and IgM) indicates that no anti-COVID-19 antibodies are detected in the specimen. The result is negative.
IgM Positive: In addition to the presence of C band, if only IgM band is developed, the test indicates for the presence of IgM anti-COVID-19 in the specimen. The result is IgM anti-COVID-19 positive.
IgG Positive: In addition to the presence of C band, if only IgG band is developed, the test indicates for the presence of IgG anti-COVID-19 in the specimen. The result is IgG anti-COVID-19 positive.
IgG and IgM Positive: In addition to the presence of C band, both IgG and IgM bands are developed, the test indicates for the presence of both IgG and IgM anti-COVID-19 in the specimen. The result is IgG and IgM anti-COVID-19 positive.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
ALL IN ALL THIS TEST IS PROFESSIONAL USE FOR AND IN-HOME USE UNDER REMOTE SUPERVISION OF A MEDICAL PROFESSIONAL.
POINT-OF-CARE, RAPID DIAGNOSIS, COVID-19 ANTIBODY TEST KITS
Advantages
coronavirus-rapid-test-kits-15-minutes.png
Rapid diagnosis. Results in 10 minutes.
Perform in any setting. No special equipment or skill required.
EVETHING YOU NEED TO DO THE TEST IS INCLUDED IN THE KIT
Allocate staff, space & PPE on a minute-by-minute basis.
Improve care, efficiency, and patient outcomes.
Establish walk-in and drive-thru testing spaces.
The Test
IgM-IgG-antibody-coronavirus-test.png
Finger prick. Single blood drop required.
IgM & IgG dual antibody detection.
Lateral flow immunochromatography.
Colloidal gold detection.
High sensitivity & specificity.
Low false positives and negative rates.
CLICK ON THE LINK BELOW TO VIEW A “HOW-TO VIDEO” TO SEE HOW THE COVID-19 RAPID TEST CASSETTE WORKS:
Please place an order request to reserve kits.
Submit an order request.
Order Details
Kits are for professional use only but some will guide you remotely over the phone step by step how you can use it in the comfort of your home.
Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals. Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
Please note that you will need to order a specimen self-collection kits to confirm the positive screening test which requires a requisition sheet that should be signed by your physician or one of the physicians on our telemedicine platform by clicking in the link below:
https://truemedhealthllc.com/
Or call at: 404-480-8609 to schedule at telemedicine consultation.
Also, feel free to print our requisition sheet in the link below for your doctor:
COVID19-PDLCONFIRMATION-PANEL.docx
and a confirmation specimen self-collecting kit will be sent to your home with a paid return shipper label.
PLEASE, SEE PREVIOUS VIDEO ABOUT SELF-COLLECTING SWAB.
Note that the self-collected specimen should not be shipped/returned on friday in order to avoid shipping delay greater than 72 hours.
Note that the customers read the disclaimers and understood the conditions that PDL should be held harmless, and they should call back for a qualified professional to show them how to use the test kits under the terms of purchase.